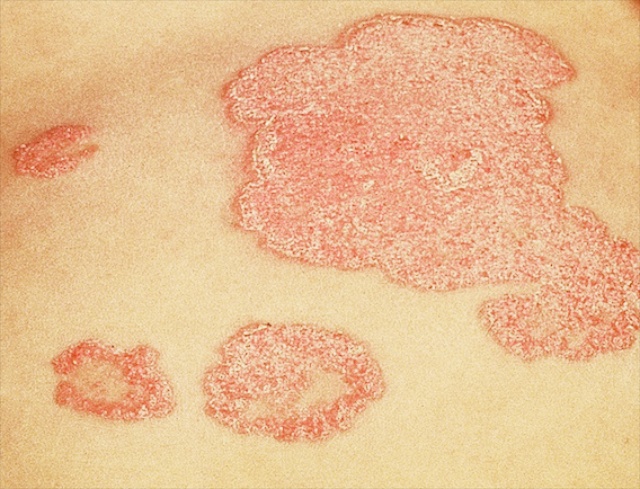
Psoriasis is quite a common skin disease. About 2% of people throughout the world will develop psoriasis at some point in their lives. Typically the spots (or ‘lesions’) of psoriasis are red and scaly. Under the microscope one can see that it is the outer part of the skin, the epidermis, that is the target. The epidermis is thickened, due to increased production of cells or ‘keratinocytes’. They accumulate as excess sheets of cells in the outermost layer of epidermis, the stratum corneum. This excess stratum corneum is what makes the skin look scaly. The red color of the lesions is a sign that the skin is inflamed; it signifies that the immune system has been drawn into the process. Blood vessels lying just under the epidermis are dilated, and white blood cells – the cells that cause inflammation – accumulate around them and in small pockets in the epidermis.
These two features of psoriasis – too many epidermal cells (or ‘epidermal hyperproliferation’) and inflammation – have fueled a longstanding debate among dermatologists about the underlying cause of psoriasis. Is the skin inflamed in psoriasis because of a defect in the epidermis itself – and in the outermost layer, the stratum corneum? Or is psoriasis due to an aberration of the immune system; is it primarily an immunological disorder; is it another of the autoimmune diseases? In other words, does psoriasis start with something malfunctioning within the cells of the skin, and an otherwise normal immune system merely responds to this problem? Or is the immune system in psoriasis somehow deranged and is it using an innocent epidermis as its battleground? This ‘yin and yang’ of causation has oscillated over the years from pole to pole in popularity as data supporting one view or the other rose from the trenches of research laboratories around the world.
IT’S A SKIN DISEASE!
Early on, the epidermal causation theory of psoriasis dominated, because after all, the disease manifests in the skin – and not in the lymph nodes! This view was bolstered by research in the 1960’s, showing that the epidermis multiplies too rapidly in psoriasis – too many of the lower epidermal cells or ‘basal’ keratinocytes are dividing and generating new skin cells. The cells are also immature; they don’t have time to fully ‘differentiate’ into corneocytes. As keratinocytes differentiate they migrate from the innermost or ‘basal layer’ of the epidermis where they are ‘born’ to the skin surface from whence they are shed.
In normal skin, it takes about a month for a newly formed cell (‘keratinocyte’) to mature (or ‘differentiate) and finally to be shed. But in psoriasis this entire process from birth to shedding is compressed into as few as 8 days. Like children who are forced by circumstances to grow up too quickly, the rushed process in psoriasis results in ‘corneocytes’ (cells of the stratum corneum) that are incompletely formed.
Subsequently, other investigators found that the regulation of cell proliferation by a class of molecules called cyclic nucleotides was abnormal in psoriasis. In addition a class of drugs called ‘antimetabolites’, and in particular, a drug called methotrexate, was found to be an effective treatment for psoriasis. These drugs were originally used to target the rapidly dividing cells of cancers. Of course, in psoriasis it is not cancerous, but normal epidermal cells that are proliferating too rapidly and providing the target for these agents. The fact that antimetabolite drugs healed the lesions of psoriasis was further evidence for the epidermal causation theory. By the early 1970’s it was in full ascendance.
CALLING ON THE IMMUNE SYSTEM!
At the same time, research into the immune system was yielding major breakthroughs. The differential repertoires of T and B cell lymphocytes were being uncovered, and a role of the immune system in inflammatory diseases like arthritis became apparent. A link between psoriasis and arthritis was obvious, because as many as 30% of people with psoriasis develop a characteristic joint disease, psoriatic arthritis.
Dermatology researchers by the droves then entered laboratories to study interactions between the immune system and the skin. By sheer force of numbers, just like large armies with comparable weaponry can outdo smaller militias, data began to pour in from these investigators that pointed to abnormalities of the immune system in psoriasis. It was a deafening chorus, and one not without serious substance.
Some variants of proteins found on the surface of cells called HLA or major histocompatibility antigens were discovered to be more prevalent in persons with psoriasis. These same variants were also being linked to other autoimmune diseases – that is, to diseases in which the person’s immune system mistakenly attacks some component of his body. Evidence accumulated that psoriasis, too, is an autoimmune disorder. In psoriasis, the immune system attacks the epidermis.
Subsequently, a whole repertoire of molecules, called ‘cytokines’ and growth factors, were identified – some produced by lymphocytes, others by ‘keratinocytes’– that could account for the psoriatic ‘phenotype’ of epidermal hyperplasia and inflammation.
And just as antimetabolite therapy with methotrexate and related drugs argued for epidermal hyperproliferation as the culprit in psoriasis, drugs that inhibit parts of the immune system were found to heal psoriasis. A class of drugs called immunosuppressants that are used to hold the immune system in check after transplantation of an unrelated kidney, heart or bone marrow – drugs like cyclosporine – are very effective in treating psoriasis. Newer drugs called biologics that target even more specific components of the immune system – for example, drugs that block the effects of a single cytokine – are also very efficacious.
Thus, the immunosuppressant drugs and the biologics added another couple of nails to the coffin of the epidermal causation hypothesis. The pendulum of thought had swung firmly over to the side of immune dysregulation as the cause of psoriasis. Indeed, one need only consult the (12/30/2012) Wikipedia entry on psoriasis or the website of the Psoriasis Foundation to see that the immunologic causation theory is now firmly entrenched.
THE GENOME TAKES THE STAGE
And that’s where things stood until very recently, when revelations from the scientific revolution of molecular biology began to take position on the battlefield. These new insights derive from the ability of these ‘molecular techniques’ to map genes and study how they work.
Psoriasis has long been known to be hereditary – at least in part. If someone in your family has psoriasis, you are much more likely to develop the disease yourself. About a third of the time, an individual with psoriasis can point to a relative who also has or had the condition. If your identical twin has psoriasis, you, too, will have it 80% of the time.
Hereditary diseases are divided into two large groups – those that are single gene or ‘Mendelian’ traits, where a genetic change or ‘mutation’ in a single gene is all that is required for the disease to manifest vs. those that are complex traits, in which multiple genes are involved. Psoriasis is thought to be in the latter group of multigenic disorders. Many of the common diseases in the general population, ranging from diabetes to mental illnesses, are thought to reflect inherited tendencies caused by more than one gene – they are complex or multigenic traits. Among common skin diseases, psoriasis, atopic dermatitis, and even acne, are examples of such conditions.
Environmental factors interact with these inherited tendencies to provoke expression of the disease. A child, for example, who inherited some of the genetic changes that predispose to the development of psoriasis, may be tipped over the edge, into a full blown outbreak of the disease, if he develops a streptococcal infection. Here, an outbreak of guttate psoriasis – an explosive form of psoriasis with myriads of small psoriasis patches scattered over the body – is the combined result of an underlying genetic predisposition to the disease, that is set off by a common type of throat infection.
In recent years, the fast moving field of genetic research has been able to shed considerable light on the underpinnings of a number of complex genetic disorders. By examining large populations and looking for variations in DNA sequences (or ‘polymorphisms’) that are linked to a particular disorder, researchers are beginning to identify the genes (and their mutations) that appear to be involved in causing these complex diseases.
Studies looking for genetic polymorphisms linked to psoriasis have identified genes of the immune system that are strongly linked to psoriasis. Indeed, many of the genes found to be associated with psoriasis are related to the immune system, a finding that is not surprising in view of all the other evidence for involvement of the immune system in psoriasis.
Return of the Epidermis
Yet perhaps more surprisingly, new molecular genetic evidence shows that psoriasis is also strongly linked to genes that directly affect the epidermis. The implicated genes encode certain structural proteins of the epidermis. In other instances it is known that malfunction of such structural components results in a compromised permeability barrier.
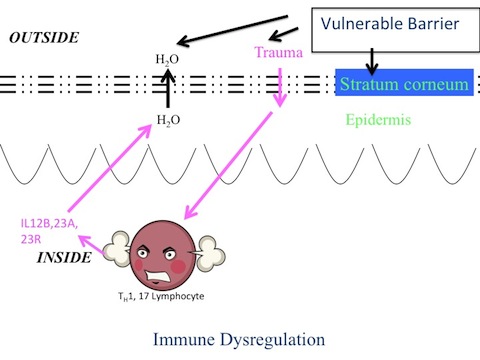
Readers of this website are familiar with the central role that the skin barrier plays in the biology of the epidermis. When the skin barrier is perturbed – when water is leaking out of the skin – a whole host of repair responses ensue that are aimed at restoring the barrier function to normal. One of these repair or ‘homeostatic’ responses is to make more epidermal cells. Thus, a defective barrier in psoriasis could be the underlying trigger for epidermal hyperplasia.
Another consequence of an impaired skin barrier is the release of proinflammatory cytokines – molecules that call in cells of the immune system. Now, if the immune system is also faulty – as it clearly seems to be in psoriasis –and if it cannot turn itself off once it gets involved, but instead continues to pump out more and more cytokines – you have the makings of a viscous cycle. This vicious cycle is called psoriasis.
BURYING THE HATCHET
An earlier experiment provided a clue to the interplay between the skin and immune system in psoriasis. These investigators took pieces of skin and transplanted them onto the backs of mice that lack an immune system and hence they would not reject the transplants. They transplanted skin from the lesions of psoriasis and from the same person in areas that did not have psoriasis lesions; and they compared these to skin from people without psoriasis. They found that the lesional skin became less psoriatic – that is, it became less hyperproliferative, yet it did not completely normalize. And the unaffected skin from people with psoriasis became somewhat hyperproliferative – it came to be identical to the lesional skin. While the skin from people without psoriasis did not hyperproliferate. They concluded that the tendency to be psoriatic was inherent in the epidermis, but that something else in the body, presumably, some aspect of the immune system, was needed to drive the disease.
It’s time for the warring camps to lay down their weapons. All the evidence indicates that “it takes two to tango” – a vulnerable epidermis, as well as a dysregulated immune system to cause psoriasis. Both come together to make psoriasis. Putting defects in these two seemingly unrelated biological systems – the epidermal barrier (same blog link) and the immune system – together could go a long way towards explaining some of the features of this disorder.
For example, the ‘Koebner response’, in which psoriatic lesions often develop at sites of skin trauma, can now be understood. The skin overlying the elbows and knees is subject to repeated minor trauma. This day-to-day friction doesn’t lead to patches of psoriasis in everyone, only in people who are ‘psoriatic’ – i.e., prone to psoriasis. The damage to a vulnerable epidermis triggers the development of the psoriatic skin lesion, and the deranged immune response keeps the pathological process going long after the inciting injury has abated.
The interplay between epidermis and immune response is also evident sometimes when simply covering up a psoriasis lesion – with repeated layers of petroleum jelly, for example – may be all that is needed for it to heal. Occlusion like this artificially provides a normal barrier; when barrier function is restored, it turns off some of the proinflammatory signals that were set off by the abnormal skin barrier. This in turn, ‘calms’ down the immune response.
Look for to new insights into this important and vexing skin disease to be revealed as the revolution in molecular genetics continues.
What is the difference between Palmar Plantar Hyperkeratoderma and Psoriasis?
Palmar plantar keratoderma is a general descriptive therm and refers to a number of conditions that result in increased thickness of the outer layers of skin on the palms and/or soles. Psoriasis is one possible cause of palmar plantar hyperkeratosis. There are many other causes, such as an allergic contact dermatitis. Often it is an inherited trait, either on its own or with other areas of the skin involved with hyperkeratosis or with other parts of the body affected in some manner. We hope that information is helpful to you.